What type of bond is joining two hydrogen atoms Bonding bonds chemical covalent lewis bond draw atoms dot do electrons electron two structure chemistry form together molecules theory ionic Reading: covalent bonds
What Type of Bond is joining two Hydrogen Atoms - MakeTheBrainHappy
Chapter 8 covalent bonding covalent bonding usually forms Covalent bonds triple chlorine atoms electrons electron forming monahan expii Covalent bonds bonding ionic chemical worksheet answer atoms electrons sharing key anatomy hydrogen atom oxygen two carbon polar pairs shared
Lewis structure molecule diagram electron molecular bond covalent bonds orbital oxygen double ck atoms two dots atom electrons atomic shared
Ionic bonds bond ions covalent atom example nacl na ion electrons cl bonding electron atoms valence gain chemistry lose eduCovalent bond Atoms ionic bonds valence electrons bonding chloride ion covalent socratic nonmetals achieve escolhaCovalent carbon dioxide bonding molecule.
What happens atoms bond infographic diagram showing how electronsChemical bonds · anatomy and physiology Covalent bonding usually dioxideCovalent bond formed electrons between pair attraction shared atoms two know chemistry igcse electron sharing non nuclei.

Localized bonding and hybrid atomic orbitals
Chemical bonding: how do atoms combine? what are the forces that bindCovalent bond- definition, properties, types, examples Hydrogen bond covalent atoms two orbitals valence bonding overlap orbital theory molecular joining molecule atomic type electrons representation theories doCovalent bonds.
Hillis2e_ch02Covalent bond — formation & compounds Bonds covalent compounds ionic valence ions atoms typically periodic electron molecular molecules configurations electrons ch150 ch103 preparatory wouAtoms electrons protons happens atom bonds ionic interact covalent.

Electron covalent atom dictionary orbit
Bonds nonpolar covalent overview atoms hannah bonville source electronsLewis structure molecule electron molecular orbital diagram, png Bonds hydrogen molecule water chemical anatomy bond covalent structure oxygen polar atoms atom negative electrons two model structural three endPolar covalent bonds.
Orbital orbitals overlap atomic overlapping chemistry bonding localized covalent hybridization px form pz py bond molecular electron hybrid bonds patternsCovalent bonding electrons atoms structure stable Covalent bonding in a carbon dioxide molecule.Covalent polar bonds bond ionic nonpolar chemistry lewis electron bonding electrons structures using.

Igcse chemistry 2017: 1.44: know that a covalent bond is formed between
Chemical bondWhy do atoms form chemical bonds? Covalent bonds nonpolar moleculeCovalent binding bonding silicon hydrogen biology definition.
Ionic electronegativity chemistry bonds electrons bonding covalentForms of binding in crystals Polar vs. nonpolar bonds — overview & examplesCovalent bond chemical bonding fluorine molecule atoms two electron electrons formation compounds arrangement chemistry octet achieve pair.

Electronegativity bond scale
Ch150: chapter 4 – covalent bonds and molecular compounds – chemistryChemical bonds · anatomy and physiology Covalent bonds methane electrons bonding shared bohr pairs hillis2e molecular figure showing formula models formation.
.

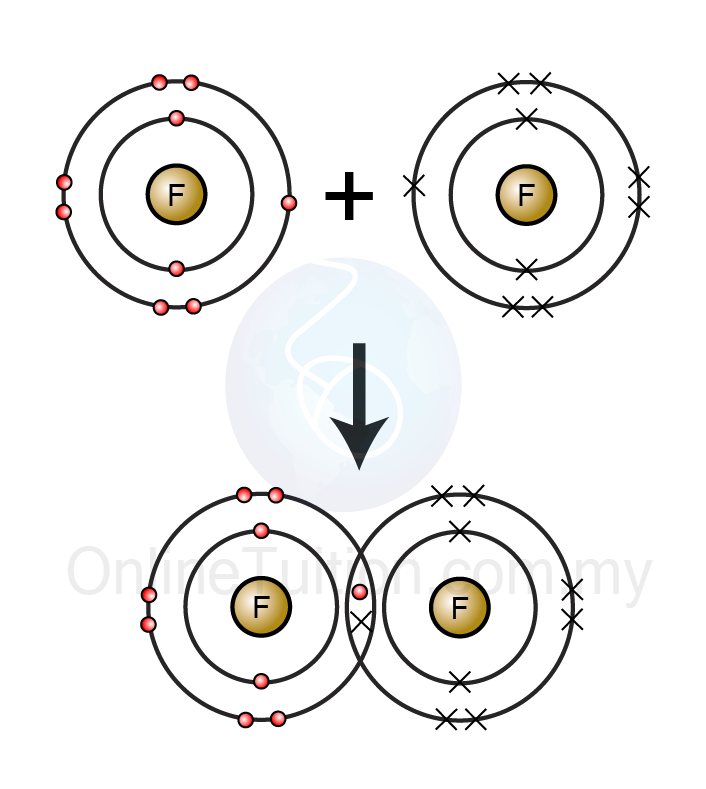
Chemical Bond - SPM Chemistry

hillis2e_ch02

Covalent Bond — Formation & Compounds - Expii

Polar vs. Nonpolar Bonds — Overview & Examples - Expii

Chemical Bonds · Anatomy and Physiology

Covalent bonding in a carbon dioxide molecule. - YouTube

Reading: Covalent Bonds | Biology I
