Explain why cation are smaller and anions larger in radii than their Building the world – be prepared! everything you should know for 1st How does ionic radius change across a period?
PPT - Ch. 9 PowerPoint Presentation, free download - ID:2090637
Anion cation ions chemistry Cation anion vs between differences definition examples ion example sources major references Cations anions atoms corresponding
Page not found
Anions ch ion no3 ppt powerpoint presentationExplain why cations are smaller and anions larger in radii than their Atomic periodic anions cations properties trends table elements size radius atom chem libretexts chemistry similarities ucd thornton jessica courtesy figureCation vs anion- definition, 10 major differences, examples.
Cation anion difference cations anions laboratoryinfo mnemonic opposite attract ionic therefore attracts attractionAnions negative periodic table ppt powerpoint presentation atoms corresponding ions larger than their slideserve Anion cation number atomic mass ppt onlineSmaller larger cation radii atoms anions.
![IONS - CATION & ANION [ AboodyTV ] Chemistry - YouTube](https://i.ytimg.com/vi/EIAaGHK5pjA/maxresdefault.jpg)
Difference between cation and anion
Atomic number, mass number and isotopesAnion cation periodic electron Radius ionic atom anionCation vs anion: definition, chart and the periodic table.
Radius ionization ions ion atom electron ecampusontario pressbooksParent explain cations atoms anions Why are cations smaller and anions are larger than the corresponding7.5: atomic properties and periodic trends.
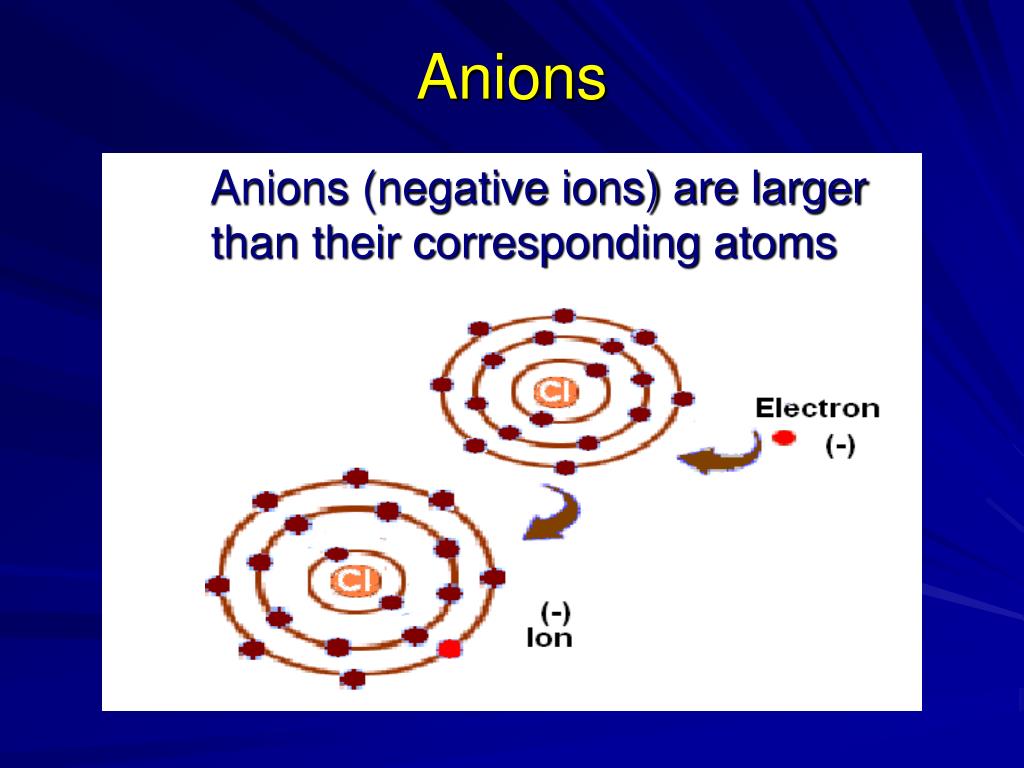
Ions sizes atoms periodic table radii atomic elements circles indicate shown chemistry colored gray
.
.


Cation vs Anion- Definition, 10 Major Differences, Examples

Building the World – Be Prepared! Everything you should know for 1st

Difference between Cation and Anion - Laboratoryinfo.com

How does ionic radius change across a period? | Socratic

7.5: Atomic Properties and Periodic Trends - Chemistry LibreTexts

PPT - Ch. 9 PowerPoint Presentation, free download - ID:2090637

Why are Cations smaller and Anions are larger than the corresponding

Cation vs Anion: Definition, Chart and the Periodic Table | Technology

Explain why cations are smaller and anions larger in radii than their
