Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry Electrons unpaired carbon bonds covalent does participate atoms valence Reading: covalent bonds
John Dalton: The Man who Ushered in Atomic Research - Math! Science
Chemical elements covalent bonding nomenclature ppt powerpoint presentation bonds form why do atoms Pi sigma bonds Elements and chemical bonds
Covalent bonds nonpolar molecule
Question video: identifying types of chemical bondingBond elements chemistry do ppt powerpoint presentation Covalent bonds chemicalBonds types chemical chemistry different.
Ionic compounds bonding stability elements ppt powerpoint presentationIonic bond bonds sodium between chloride difference ion covalent metallic forces examples interactions intramolecular formation compounds types properties bonding atoms 14.1 sigma and pi bonds (hl) old versionCovalent molecules compounds chemistry bonds molecular elements introduction.
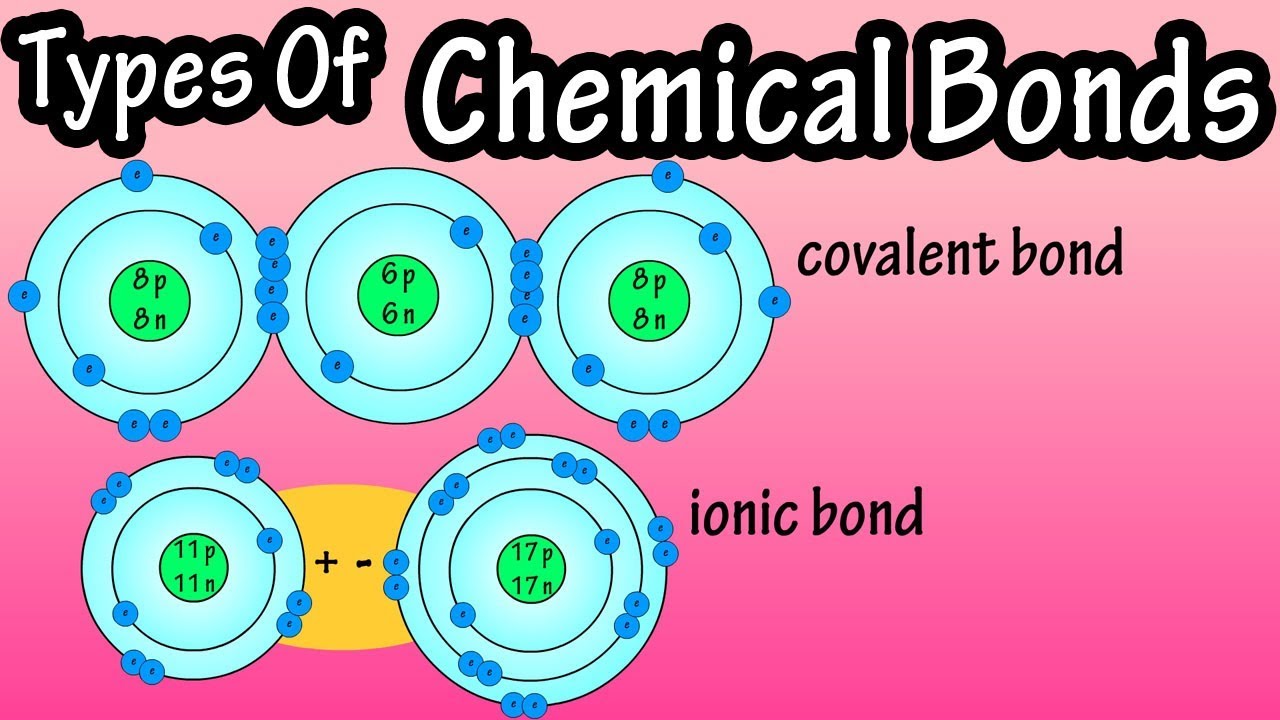
Types of chemical bonds
John dalton: the man who ushered in atomic researchHow many covalent bonds does carbon form if each of its unpaired Dalton john atomic ushered research man who theory sameElements compounds form substances.
Chemical bonding nagwaBonds chemical types ionic covalent ions Ion-ion interactionsChemistry lesson.

Covalent bond: definition, types, and examples
.
.


PPT - Covalent Bonding and Nomenclature PowerPoint Presentation, free

Reading: Covalent Bonds | Biology I

PPT - Chemistry 120 PowerPoint Presentation, free download - ID:2089971

How many covalent bonds does carbon form if each of its unpaired
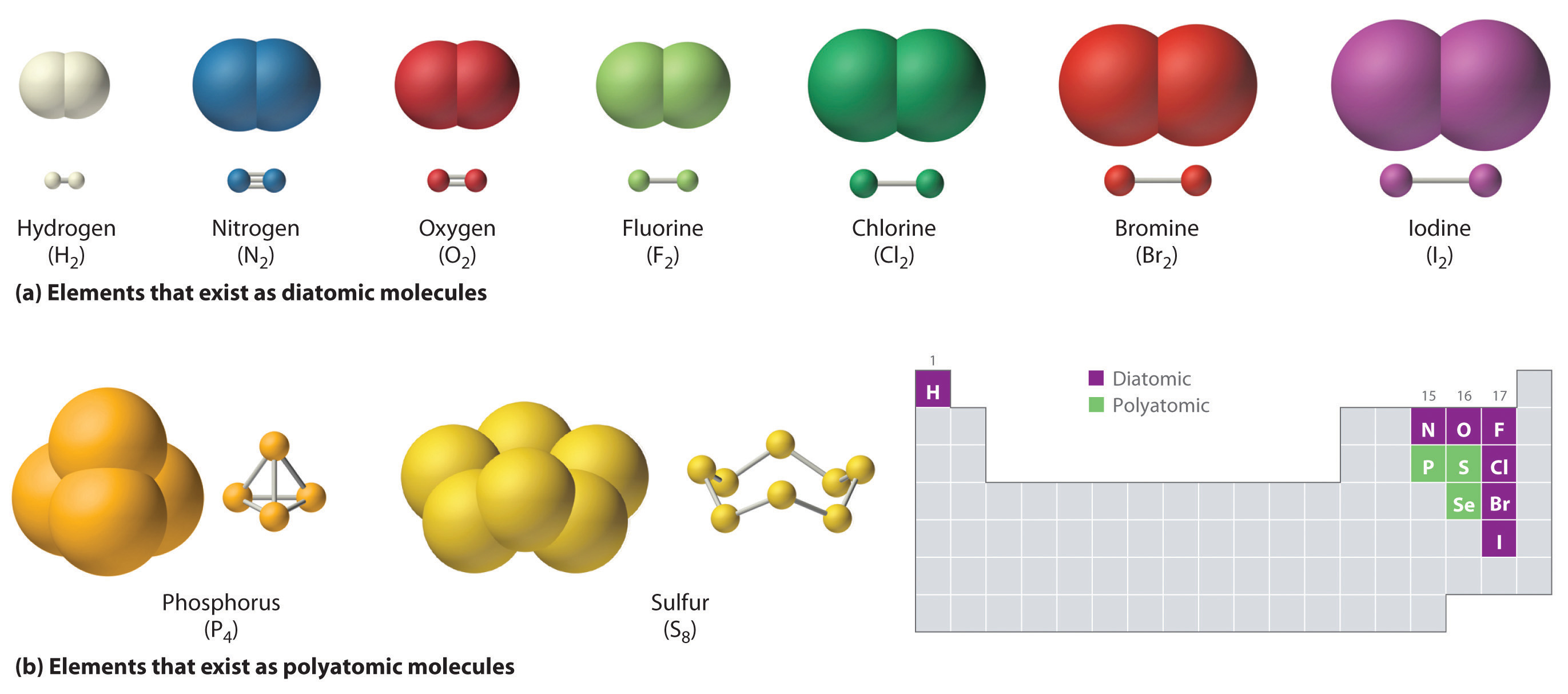
CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

14.1 sigma and pi bonds (HL) old version - YouTube

Covalent Bond: Definition, Types, and Examples

Elements and Chemical Bonds

Ion-Ion Interactions | Brilliant Math & Science Wiki
